Steam Distillation
By Maryellen Nerz-Stormes, Ph.D.
A new technique that arises in this experiment is that of steam distillation. When you isolate the clove oil (eugenol and acetyl eugenol) from cloves, you will not have a solution. Instead, you will have two layers. Unlike the distillation of a solution (e.g., cyclohexane/toluene), these two layers will behave as distinct entities and there will be no dependence on how much of each species is present. The total pressure of the pot liquids can be defined by the following equation.

Notice there are no mole fraction terms in the equation. This means that if you have lots of water or just a little it will make the same contribution to the vapor pressure. What will happen when you distill? The mixture will heat up and eventually boil. Please recall that boiling occurs when the pot liquids have a vapor pressure equal to the external pressure. In steam distillation, the pressures of the two components must add up to 760 torr. Throughout the heating process, water and clove oil molecules will escape in proportion to their respective vapor pressures at the distilling temperature. Since water has a significantly lower boiling point than eugenol or acetyl eugenol, a much greater proportion of water molecules will be vaporizing at any time during the distillation. Even though the components of clove oil have low vapor pressures, they are volatile enough to vaporize to some extent and a small amount will lift off with the water molecules. Since the water and organic components are not interacting with each other, no enrichment occurs and they will co-distill at a single temperature until all of one component is completely distilled over. Normally, steam distillations are carried out with a large excess of water. When all the organic component has been distilled, pure water begins to distill. How is this situation reflected in the appearance of the liquid and in the still head temperature?
While the steam distillation is occurring, the boiling point of the two together will be lower than the boiling point of the more volatile component. Why? At the end of the distillation, you will have two layers in the receiver which can be separated.
A helpful relationship when considering steam distillation in a theoretical sense is the ideal gas law, PV = nRT, where P = pressure, V = volume, n = moles, R = the gas constant and T = temperature. It is important to remember that all of these parameters refer to gaseous molecules. Since distillation involves the expansion of a liquid into a gas in a fixed volume (the still), the gas law can be useful in predicting the amount of water needed to complete a steam distillation or to figure out the proportion in which the organic and aqueous layers will co-distill. To gain a more practical expression, take the ratio of a gas law written for the gaseous water and one written for the organic gas. If this is done, one obtains the following expression.
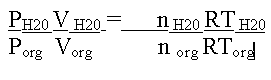
Fortunately, several of the terms in the above expression cancel. The volumes cancel because both gases occupy the same space, i.e., the still. The temperature terms cancel because the two components are co-distilling at the same temperature. The R terms obviously cancel.
The equation therefore reduces to:
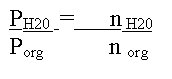
This simple equation sums up steam distillation because it demonstrates that the amount of water obtained is directly proportional to the vapor pressure of water at the distillation temperature. The same is true of the organic component . Therefore, if the organic component has a higher boiling point than the aqueous component, it will contribute fewer molecules to the overall push against the atmosphere. Nonetheless, the two components are working together. You can think of the system as being like two people trying to push a broken down car. The weaker person may not be contributing much, but is still reducing the work for the stronger person. Because the organic is there, the water does not have to push as hard against atmosphere and this is why the overall temperature is the below the boiling point of pure water.
Now with all this sophisticated theory stated, why is steam distillation useful? You might wonder why you would not just take the cloves and press the oil out of them or extract the cloves directly with an organic solvent such as methylene chloride or ether. The problem with pressing the oil out (and this can be done) is that the yield is very low. You might be able to imagine that much of the material would get caught up in the solid matrix that constitutes most of the mass of the cloves. The problem with a direct organic extraction is that many other nonvolatile organic components of the clove would also dissolve in the solvent resulting in a much more complex mixture. Purification would become time consuming and material would lost with each added step. With steam distillation, only the volatile components are collected and they can be isolated exhaustively if enough water is used.
In summary, steam distillation is an ideal way to separate volatile compounds from nonvolatile contaminants in high yield. For these reasons it has been used extensively in the isolation of natural products.
[Study Aids]