By Eliana Saxon and
Maryellen Nerz-Stormes [Back to Study Aids]The human tongue can recognize only four basic tastes (sweet, salty, sour and bitter) while the receptors in the nose are able to discriminate among seven distinct smells, camphoraceous, musky, floral, pepperminty, ethereal, pungent and putrid. Each of the first five scent classifications are associated with fairly distinct molecular shapes and dipole moments allowing each to interact specifically with their corresponding smell receptors. The molecules giving rise to the putrid and pungent odors only seem to have a dipole moment dependence. .
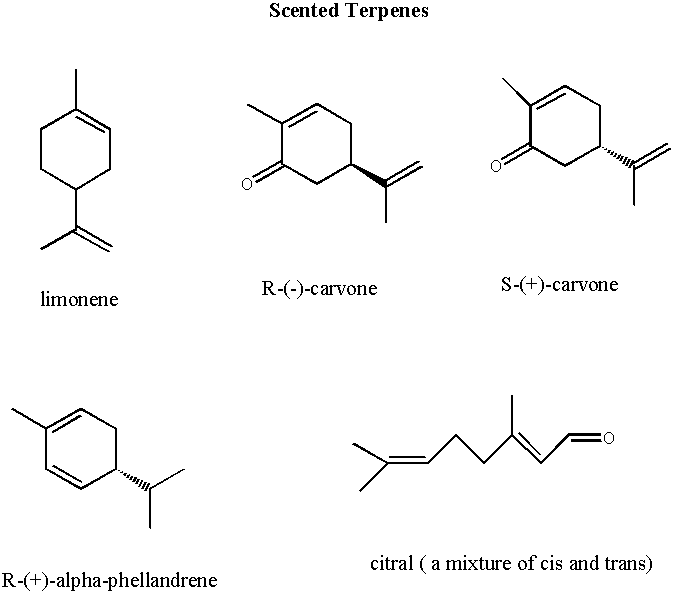
The following pages give the structures and corresponding names of a number of scented compounds. You can test the scents of most of these compounds while you are in lab this week. Though conservative scent testing of compounds is encouraged this week, the general lab rule is to avoid organic fumes.
The most important concept to get out of this reading and your experience in lab is that scent is related to structure. Though we may not totally understand what it is about the structure that causes us to perceive a particular scent or the exact biochemistry of the sensation, we can say conclusively that the different scents of compounds are due to specific aspects of their structure. In fact, all physical properties are directly related to molecular structure. It is particularly noteworthy that the two carvones have such different scents!!!! These compounds are known as enantiomers. They are very closely related, differing only in the spatial arrangement of two groups. Specifically, they are mirror images of one another. . The fact that one carvone has a spearmint scent and the other has a caraway scent indicates that smell receptors have a handedness or chirality
Aromatic compounds received their name because many compounds that contain aromatic rings (e.g. benzaldehyde from almonds) have distinctive smells. However, it is now known that not all compounds with benzene rings have fragrance and not all fragrances have aromatic rings. In this lab you will work with a series of structurally related compounds that do contain aromatic rings and have unique fragrances.
You will be isolated clove oil in this laboratory. Clove oil makes up 14-20% of the weight of cloves. It is predominantly comprised of eugenol, but also contains a small amount of acetyleugenol. Eugenol is also found in cinnamon leaf, West Indian bay and allspice. Clove oil is easily isolated from cloves by steam distillation because it is thermally stable at the distilling temperature and volatile enough to contribute to the vapor pressure. . As mentioned previously in this lab, steam distillation is an excellent way to exhaustively isolate a volatile organic material from a solid matrix. In addition to its use as a fragrance and flavoring, clove oil has medicinal applications. It has been used as a dental analgesic for thousands of years.
Eugenol and acetyleugenol belong to a class of compounds called vanilloids. The structures of several of these compounds are shown on the following pages. Several of the vanilloids will be available for you to test for scent in lab . In addition to clove oil, vanillin (from vanilla beans), ethyl vanillin (artificial vanillin) and vanillyl alcohol ( obtained from the chemical reduction of vanillin) will also be available for comparison. Shown on the following pages, but not included for scent testing is capsaicin. Capsaicin is the compound that gives hot chili peppers their bite. Capsaicin and related compounds are being studied for their pain relieving properties and capsaisin is used in bird seed to keep the squirrels out. Anyone who has a standard bird feeder knows that squirrels are the most acrobatic and persistent creatures in the world. They can consume almost any bird feeder and its contents in a matter of days. Capsaisin is helpful because the birds don't mind the taste, but the squirrels dislike it. In this lab, it is desired that you will be able to determine the parts of the molecule that are responsible for the quantity and quality of the scents you experience.

[Back to Study Aids]