Chirality, Enantiomers and Optical Rotation
By
Maryellen Nerz-StormesIn preparation for this polarimetry exercise, please read pages 234-240 of Loudon in addition to the following passages.
As you have no doubt learned in lecture by now, when two molecules are described as enantiomers they have a very close relationship. They are stereoisomers that are nonsuperimposable, mirror images. As hard as it is to believe, the individual molecules that constitute a pair of enantiomers are distinct molecules that can in principle be separated from one another. Once separated, they do not interconvert. Interconversion requires breaking two sigma bonds which is no easy feat since the energies involved in the transformation are rather high.
Each individual molecule in an enantiomeric pair is chiral. Chiral molecules are molecules that are so asymmetric that they are nonsuperimposable on their mirror images. The term chiral can also be used to describe macroscopic objects. Similar to molecules, a macroscopic object is chiral if it can't be superimposed on its mirror image.
As mentioned previously in this manual, chirality is very important in biological systems as it is a fundamental component of molecular recognition. The concept is not unlike the experience a left handed person has in this rather right handed world. Left handed people can have some trouble when they encounter other chiral or handed objects and beings. For example, it is tough for a left handed person to take notes at a right handed desk. Of course, left handed people have no problem when they encounter achiral objects. For example, a left handed person is very comfortable when he or she is sitting at a simple table. This all changes when a righty sits down next to him or her. It is the same for molecules in a living system because living systems are filled with chiral molecules. Enantiomeric molecules interact differently with the chiral molecules they encounter. An example given previously in this course are the two enantiomeric carvones. Remember how dissimilar they smelled? They have distinct fragrances because they interact differently with smell receptors which are chiral.
Like left handed and right handed people, chiral molecules behave the same way when in achiral environments. It is for this reason that enantiomers are tough to physically separate. When they are in an achiral environment, they have exactly the same physical properties. For example, they have the same boiling points, melting points and solubilities. When you think about the comparative structures of enantiomers, it makes sense that they have identical properties. Enantiomers have the same molecular weight, connectivity and dipole moments. This means that they can not be separated directly by any of the conventional versions of techniques you have learned in this course such as distillation, gas chromatography, extraction or recrystallization.
You have probably learned in lecture that chiral molecules have optical rotations and that enantiomers rotate plane polarized light in equal and opposite directions. Plane polarized light is light that has been passed through a polarizer. Light is thought to consist of an infinite number of oscillating electrical and magnetic planes. When light passes through a polarizer, all planes but one are removed.
This behavior upon exposure to plane polarized light is not some magical property that defies the general principles of enantiomers. The reason enantiomers interact differently with plane polarized light is that plane polarized light is chiral. Though polarized light is a planar phenomenon, it is actually comprised of two superimposed helical forms. You can think of the electrical component as oscillating in such a way that there are two electrical vectors rotating in a cork screw fashion in opposite directions away from the source of the propagation at the same rate. Since a helix is a chiral shape , the two versions of the light constitute an enantiomeric pair. If you resolve the vectors of the two superimposed helices, you end up with oscillation in a single plane. This concept will be illustrated in class.
Enantiomers interact differently with the two forms of light. The basic idea is that when plane polarized light encounters enantiomerically pure, chiral molecules, one form of light will slow down more than the other as a consequence of the interaction. This results in one helix coming out of sync with the other. When the vectors are resolved, the resultant plane is rotated with respect to the original position. This change in position is what we mean when we say a compound has an optical rotation.
Since optical rotation is a consequence of asymmetry, several groups of molecules will not exhibit rotations. Obviously, achiral molecules will not rotate plane polarized light. Realize that achiral molecules include meso compounds. 50:50 mixtures of enantiomers will not give rotations. Why?
The following are optical rotations for a series of natural products.
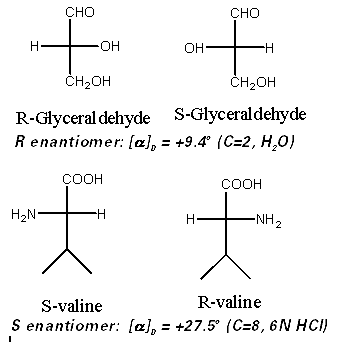
The important conclusion to draw from the above data is that there is no obvious correlation between the sign of rotation and the absolute configuration (R/S designation). Some R compounds give positive rotations and some negative rotations. There is also no obvious correlation between the magnitude of the rotation and the structure, though there must be a relationship. Once the stereochemistry is established by an independent method, the sign and magnitude of the rotation is a constant for a given chiral compound. Considering this information, can optical rotation be used to establish the absolute configuration of a newly discovered chiral compound?
The numbers reported above are called specific rotations. Since experimentally measured optical rotations are dependent on the nature of the compound, the wavelength of light, the solvent, the temperature and the number of molecules in the path of the light, the measurements have to be standardized so that they can be of universal value. Therefore, when an experimental measurement is made, the value is divided by the concentration and length of the polarimetry tube. Concentrations are reported in grams/mL and the pathlength in decimeters. The wavelength of light, the temperature of the measurement and the solvent used are always reported with the specific rotation in the literature. Most measurements are made using a sodium lamp at room temperature.
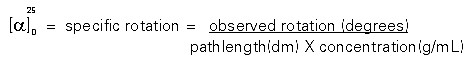
The separation of enantiomers is called resolution. As mentioned previously, it is not easy to separate enantiomers because they have the same physical properties in achiral environments. The first resolution was achieved by Louis Pasteur over one hundred years ago. As described in your text book (Loudon, pp. 261- 262), Pasteur studied a crystalline mixture of enantiomeric sodium ammonium tartrate salts under the microscope, and he observed that there were two different crystal forms that had chiral shapes and were enantiomeric with respect to each other. He painstakingly separated these two forms and discovered that the separated forms had equal and opposite rotations.
When one has a pair of enantiomers in a 50:50 mixture, it is called a racemate or racemic mixture. As mentioned previously, racemates do not have an optical rotation. Racemates are said to have zero percent optical purity. Resolutions are often only partially successful, meaning a mixture of enantiomers is isolated in which there is more of one enantiomer than the other. These mixtures have partial optical purity. If only one form of the enantiomeric pair is isolated, the compound is said to be optically pure or to have 100 % optical purity. The following formula is used to calculate optical purity. In this formula, the experimental specific rotation is compared to the literature value. If the resulting optical purity is less than the literature value, but more than zero some level of optical purity has been achieved. The meaning of specific optical purity values will be discussed in class.
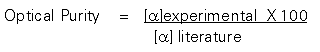
You might wonder why resolution is significant and why optical purities and rotations are measured. One application is particularly effective at illustrating the importance of chirality and optical purity. Most drugs that are in use today are chiral molecules, many of which are natural products or derivatives of natural products. A drug that is a natural product is a compound isolated from a plant or an animal that exhibits some sort of biological activity. In other words, we get a lot of our inspiration for drug design from mother nature. Conventional synthesis of chiral molecules results in the formation of racemic mixtures or racemates, whereas the natural products usually exist in optically pure form. If a racemate was administered to a human subject to treat some disease, the two forms might not behave the same way in the chiral world of the human body. Those enantiomers would encounter many different chiral molecules and would not necessarily interact with them in the same or even a beneficial way. For this reason, it is essential for most drug synthesis to result in optically pure material. One way to achieve optical purity is through resolution. Another is through chiral synthesis. In other words a single enantiomer can be made by doing the synthesis in a chiral environment. Increasingly, chiral synthesis is induced using natural enzymes.
Pasteur's resolution was rather fortuitous. Normally, enantiomers do not form mirror image crystals. So how do scientists more routinely separate enantiomers? One way is to carry out some sort of chemistry that changes the relationship between the molecules, but does not change the fundamental stereochemistry of the original molecules. Consider for example, taking an R/S pair of enantiomers and reacting it with an optically pure compound having the R absolute configuration. I will call this second compound R* to minimize confusion. If enough R* is added, two molecules will form, R-R* and S-R*. These simple minded designations mean that the original compound having R or S absolute configuration have been chemically bonded to another molecule having the R absolute configuration. Since by definition R is the mirror image of S and RR is the mirror image of SS, these two new molecules can't be enantiomers. They now have a more distant relationship. They are diastereomers. Diastereomers are stereoisomers that are nonsuperimposable and not mirror images. In principle, diastereomers have different physical properties and can be separated. Once R-R* and S-R* are physically separated, they are chemically transformed back to their original forms, R and S. A nice example of this type of resolution is given in your textbook on pages 249-251. In this sort of process, perfect resolution is seldom attained in one shot so usually the process has to be repeated several times on increasingly resolved material. With this in mind, the necessity of measuring optical rotations and calculating optical purities becomes more obvious.
In this exercise, partially resolved and optically pure solutions of chiral compounds will be made available to you. The only information you will be given is the concentrations of the solutions. Working with your spectroscopy group, you will attempt to determine the following. The solutions to each of the following problems will be submitted by your group to your instructor. Please only submit one solution per group. It is very, very important that all students in the group participate in all aspects of this exercise.
1. What is the correct sign and magnitude of the observed rotation. The problems associated with this issue will be discussed in class.
2. What is the specific rotation for each solution?
3. What is the optical purity of the impure solution?
4. What is the ratio of R:S molecules for the impure solution? Can you fully answer this question? If not, explain.
5. Suppose you are an extremely talented, versatile organic chemist who has collected a number of new plant species from an Amazonian rain forest. Give a rough outline of the steps you would take in developing and implementing a new drug from your biological samples.
[Return to Study Aids]